IDIAG M360® OVERVIEW
Diagnostics
Individual back scan for targeted therapies.
Optimised treatment outcome and reduced risks.
Therapy monitoring
Transparent patient information in the form of simple, easy-to-understand graphics.
Precise data on the spinal column’s geometry to document the treatment outcome.
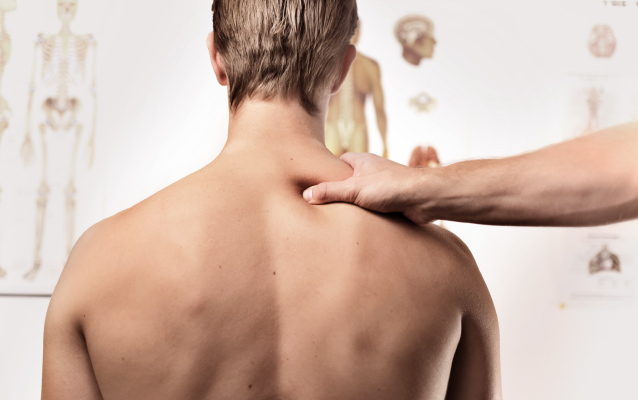

Prevention
Identification of any abnormalities via substantiated determination of back geometry, stability and mobility.
Generation of relevant clinical data for the development of successful therapies.
.
KEY FACTS
BENEFITS
More customers due to an individual back analysis. Clearly laid-out standard and individual reports show the current state of the spinal column and document the outcome of the therapy.
ORIGINS
The Idiag M360 was developed in close cooperation with Ludwig Maximilian University in Munich, Germany.
CERTIFICATION
The Idiag M360 is a CE-certified product and has been granted other international certificates.
USAGE
This product with unique functionality is used by thousands of doctors and therapists, and in around 5000 clinics and health centres worldwide.
DISTRIBUTORS
Are you looking for a distributor in your country to buy the Idiag M360?
FAQ
What is the difference between the Idiag M360, MediMouse and SpinalMouse?
After an Idiag M360 recording, what information is available to the patient?
What is the operating principle of the Idiag M360?
What do the reference values of the Idiag M360 relate to?
CONTACT
Do you have a question about the Idiag M360, do you need support or would you like to order directly?




